Chemical reactions are an important element of technology, since they contribute to a variety of human activities that we encounter regularly. The burning of fuels and the production of wine and beer are examples of chemical processes that we encounter on a daily basis. Chemical reactions may also be found in nature, such as in the chemical weathering of rocks, photosynthesis in plants, and animal respiration.
Physical, chemical, and nuclear reactions are the three types of reactions in general. Chemical reactions can be further classified into a variety of groups. Synthesis, decomposition, single-displacement, double-displacement, combustion, and acid-base reactions are six typical kinds of chemical reactions. Scientists divide them into categories depending on what occurs when they transition from reactants to products. This is useful for forecasting reagent reactivity and the products produced by reactions.
Different Types of Chemical Reactions
Here is the latest list of all types of chemical reactions that you should know about.
1. Combination Reactions
A synthesis reaction, also known as a combination reaction, occurs when two or more chemicals combine to produce a single new compound. Synthesis reactions are another name for combination reactions. A combo reaction takes the following general form:
A+B’AB
Two components combine to produce a compound in a combination reaction. When solid sodium metal interacts with chlorine gas, solid sodium chloride is formed.
2Na(s)+Cl2(g)’2NaCl(s)(5.3.2)
It’s necessary to know the seven elements that occur in nature as diatomic molecules in order to construct and balance the equation correctly (H2, N2, O2, F2, Cl2, Br2, and I2).
The interaction of an element with oxygen to create an oxide is a common example of a combination reaction. Under normal circumstances, metals and nonmetals both react easily with oxygen. When magnesium is burned, it reacts quickly and spectacularly, mixing with oxygen from the air to form a fine powder of magnesium oxide.
2Mg(s)+O2(g)’2MgO(s)(5.3.3)
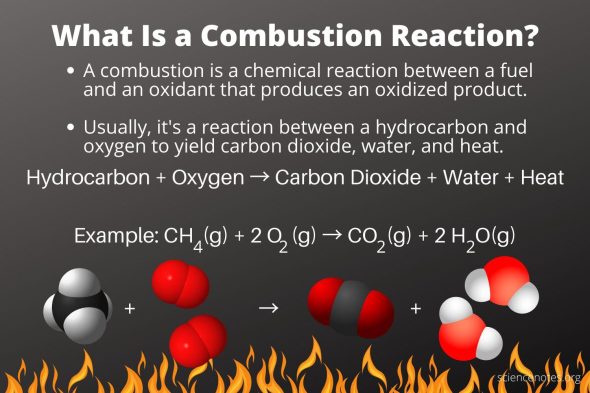
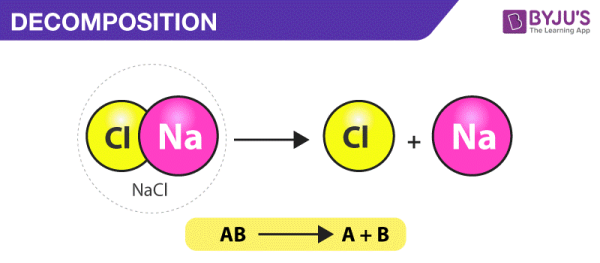
2. Decomposition Reactions
A decomposition reaction occurs when a complex material is broken down into two or more simpler compounds. A decomposition reaction takes the following general form:
AB’A+B
A source of energy, such as heat, light, or electricity, is required for most breakdown processes. Binary compounds are those that include only two components. When a binary substance decomposes into its constituent parts, it is the simplest type of decomposition reaction. When mercury (II) oxide, a red solid, is heated, it decomposes into mercury and oxygen gas.
2HgO(s)’2Hg(l)+O2(g)
Even if one or more of the products is still a chemical, a reaction is called a breakdown reaction. A metal carbonate decomposes into a metal oxide and carbon dioxide gas when exposed to air. Calcium carbonate, for example, decomposes into calcium oxide and carbon dioxide.
CaCO3(s)’CaO(s)+CO2(g)
Metal hydroxides breakdown into metal oxides and water when heated. When sodium hydroxide decomposes, sodium oxide and water are produced.
2NaOH(s)’Na2O(s)+H2O(g)
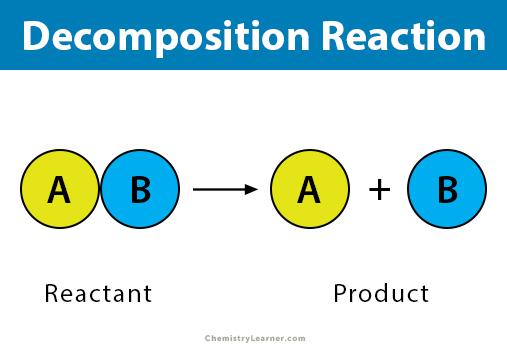
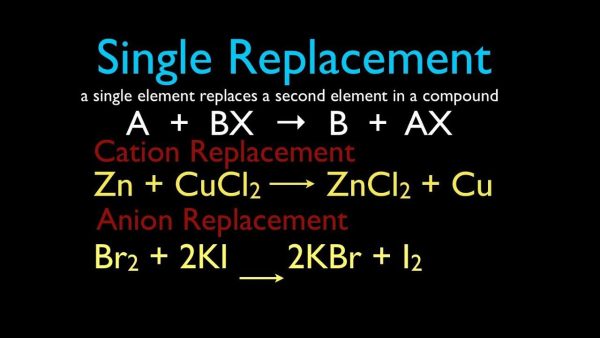
3. Single-Replacement Reactions
A single-replacement reaction occurs when one ingredient in a compound replaces another comparable element. A single-replacement (also known as single-displacement) response takes the following general form:
A+BC’AC+B
Element A is a metal in this reaction, and it substitutes element B, which is also a metal, in the combination. When the replacement element is a nonmetal, it must replace another nonmetal in the complex, then the general equation becomes:
Y+XZ’XY+Z
Y is a nonmetal that substitutes for the nonmetal Z in the compound. Magnesium is a metal with a higher reactivity than copper. When a strip of magnesium metal is immersed in aqueous copper (II) nitrate solution, it replaces the copper. The process produces aqueous magnesium nitrate and solid copper metal as byproducts.
- Mg(s)+Cu(NO3)
- 2(aq)’Mg(NO3)2(aq)+Cu(s)
Many metals are easily reacted with acids, and one of the results of this reaction is hydrogen gas. Aqueous zinc chloride and hydrogen are formed when zinc interacts with hydrochloric acid (see figure below).
Zn(s)+2HCl(aq)’ZnCl2(aq)+H2(g)
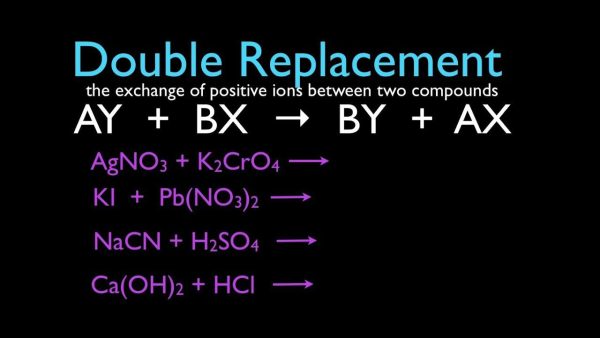
4. Double-Replacement Reactions
A double-replacement reaction occurs when the positive and negative ions of two ionic compounds swap positions, resulting in the formation of two new compounds. A double-replacement (also known as double-displacement) response takes the following general form:
AB+CD’AD+CB
A and C are positively charged cations in this process, while B and D are negatively charged anions. In most cases, double-replacement reactions happen between compounds in aqueous solution. One of the results of a reaction is often a solid precipitate, a gas, or a molecular molecule such as water.
When the cations from one of the reactants mix with the anions from the other reactant to produce an insoluble ionic compound in a double-replacement reaction, the result is a precipitate. When potassium iodide and lead (II) nitrate water solutions are combined, the following reaction happens.
- 2KI(aq)+Pb(NO3)
- 2(aq)’2KNO3(aq)+PbI2(s)
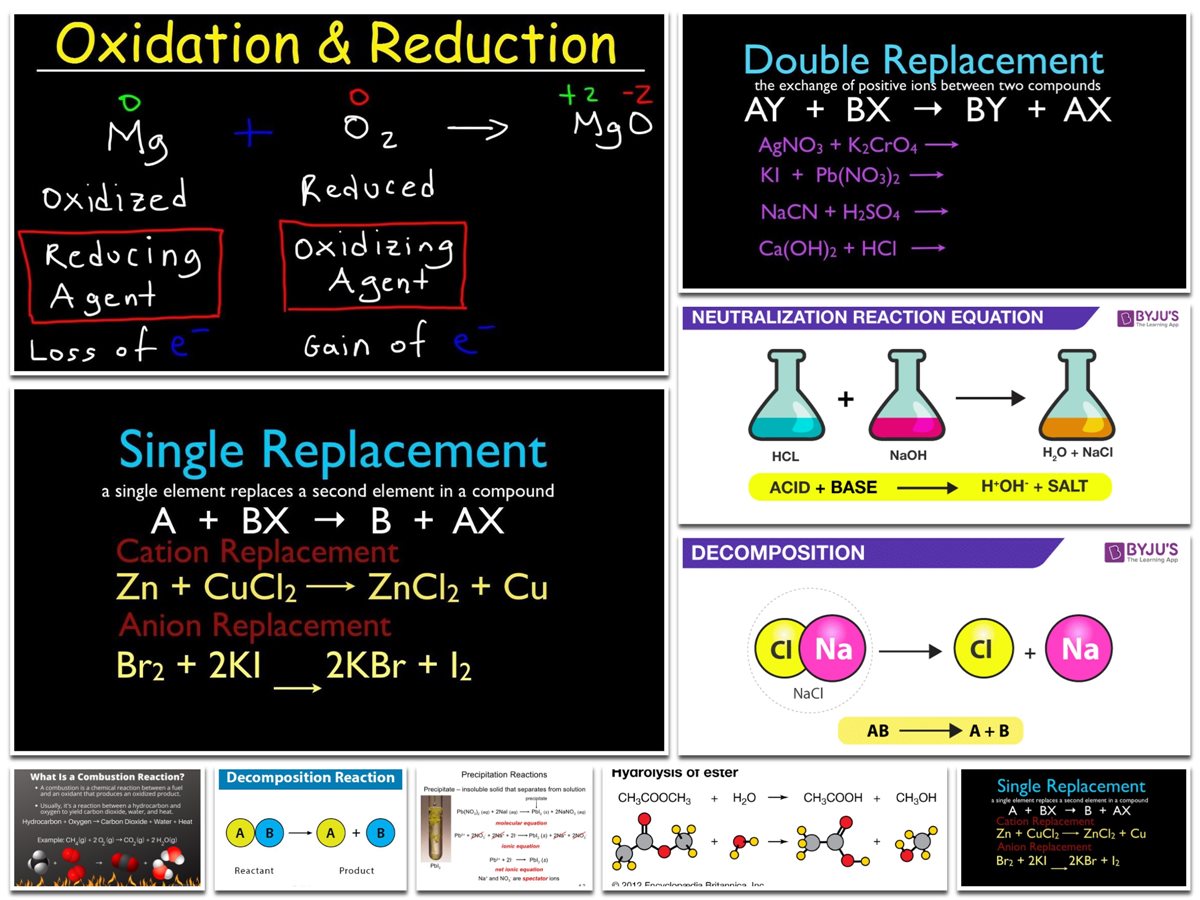
5. Combustion Reactions
A combustion reaction occurs when a material interacts with oxygen gas and produces energy in the form of light and heat. One of the reactants in combustion processes must be O2. Water vapour is produced when hydrogen gas is burned (see figure below).
- 2H2(g)+O2(g)
- +2H2O(g)
It’s worth noting that this is also a combo response. A hydrocarbon, a substance composed entirely of carbon and hydrogen, is used in many combustion processes. Carbon dioxide and water are always produced when hydrocarbons are burned. Many hydrocarbons are utilized as fuel because they produce a high quantity of heat energy when burned. Propane (C3H8) is a gaseous hydrocarbon that is frequently used in gas grills as a fuel source.
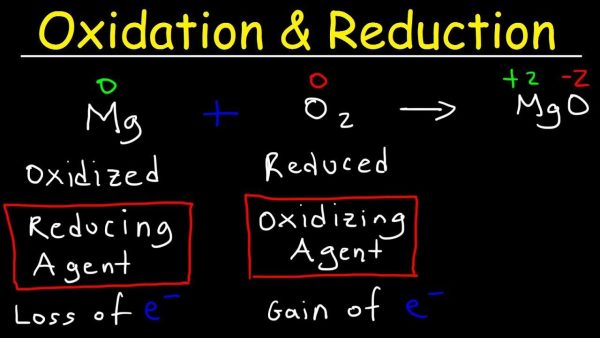
6. Oxidation-Reduction Or Redox Reaction
The oxidation numbers of atoms are altered in a redox process. The transfer of electrons between chemical species is possible in redox reactions. The following is an example of a redox reaction that happens when I2 is reduced to I- and S2O32- (thiosulfate anion) is oxidised to S4O62-:
S4O62(aq) + 2 I2(aq) = 2 S2O32(aq) + I2(aq) (aq)
7. Direct Combination Or Synthesis Reaction
Two or more chemical species mix in a synthesis process to produce a more complex compound.
AB = A + B
A synthesis reaction is the reaction of iron and sulphur to produce iron (II) sulphide:
8 Fe + S8 ‘ 8 FeS
8. Chemical Decomposition Or Analysis Reaction
A substance gets broken down into smaller chemical species in a breakdown process.
A + B = AB
A decomposition reaction is the electrolysis of water into oxygen and hydrogen gas:
2 H2O ‘ 2 H2 + O2
9. Single Displacement Or Substitution Reaction
A substitution, also known as a single displacement reaction, occurs when one element is displaced from a molecule by another.
AC + B = A + BC
When zinc reacts with hydrochloric acid, an example of a substitution reaction occurs. The hydrogen is replaced with zinc:
Zn + 2 HCl ‘ ZnCl2 + H2
Metathesis, also known as the Double Displacement Reaction, is a chemical reaction in which two molecules are
Two chemicals swap bonds or ions in a twofold displacement or metathesis process to produce new molecules.
AB + CD ‘ AD + CB
The formation of sodium nitrate and silver chloride from sodium chloride and silver nitrate is an example of a double displacement reaction.
NaCl(aq) + AgNO3(aq) ‘ NaNO3(aq) + AgCl(s)
10. Acid-Base Reaction
A twofold displacement reaction between an acid and a base is known as an acid-base reaction. Water and an ionic salt are formed when the H+ ion in the acid interacts with the OH- ion in the base:
HA + BOH ‘ H2O + BA
An acid-base reaction is the reaction between hydrobromic acid (HBr) and sodium hydroxide:
HBr + NaOH ‘ NaBr + H2O
11. Isomerization
The structural arrangement of a molecule changes during an isomerization reaction, while the net atomic composition remains the same.
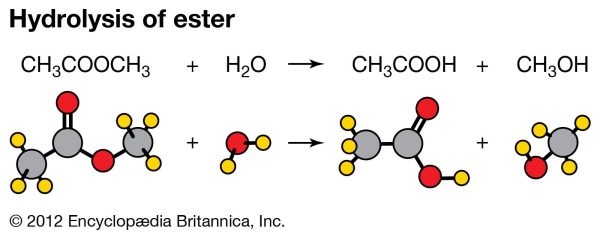
12. Hydrolysis Reaction
Water is involved in the hydrolysis process. A hydrolysis process takes the following general form:
X-(aq) + H2O(l) ” HX(aq) + OH-(aq)
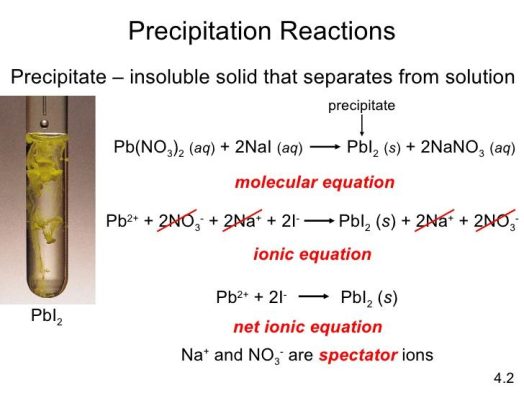
13. Precipitation Reactions
The double replacement reactions precipitation and neutralisation are both double replacement reactions. Both of these reactions result in the formation of two totally new compounds as a result of double replacement. When two soluble chemicals combine to produce an insoluble solid, this is known as a precipitation reaction. The precipitant is the solid that separates from the solution. Silver nitrate reacts with potassium chloride to create silver chloride, a white solid, which is a famous example of a precipitation reaction.
ch{AgNO3(aq) + KCl(aq) -> AgCl(s) + KNO3(aq)}
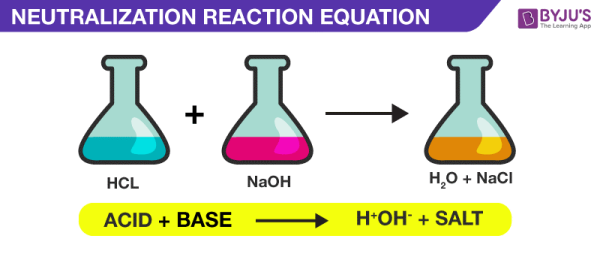
14. Neutralization Reactions
The reaction between an acid and a base is the other sort of double-displacement reaction. Water is formed via this double-displacement process, known as a neutralisation reaction. Take a look at the sulfuric acid (car battery acid) and sodium hydroxide mixing solutions (lye).
Frequently Asked Questions (FAQs)
How Can You Tell Whether A Chemical Reaction Is Occurring?
Physical indicators of a chemical reaction, such as heat and light emission, precipitate formation, gas evolution, or a change in appearance, are usually visible.
What Methods Do You Use To Spot Physical And Chemical Changes?
A physical transition changes the shape or form of matter, but not the kind of substance in the material. On the other hand, a chemical shift changes the type of matter, resulting in the creation of at least one new substance with new characteristics. The gap between physical and chemical transitions does not have a clear cut.
What Is The Purpose Of Writing A Chemical Equation?
The goal of constructing a balanced chemical equation is to explain the reactants (beginning material) and products that occur throughout the reaction (end results). The ratios in which they respond so that you can calculate the number of reactants you’ll require and the number of commodities you’ll be able to produce.
What Is The Difference Between A Chemical Reaction And A Chemical Equation?
A chemical equation is a symbolic representation of a chemical reaction in the form of symbols and formulae, with the reactant entities on the left side and the product entities on the right.
What Is The Skeleton Equation, And How Does It Work?
A skeleton equation is one in which each product involved in the reaction is represented by the chemical formulae that describe the reaction. Example: The following is the term equation: oxygen + methane. Carbon dioxide + Vapour = Dioxide.