Dmitri Mendeleev, a Russian chemist, presented his first effort at categorising chemical elements according to their atomic weights in the late 1800s. Mendeleev noticed that when the elements were grouped by weight, some sorts of elements appeared in regular intervals, or periods.
Today, scientists officially acknowledge 118 elements (with the inclusion of four newcomers in 2016) and continue to categorize them using Mendeleev’s periodic table of elements. The table begins with the most basic atom, hydrogen, and then arranges the remainder of the elements by their atomic number, or the number of protons they contain. The sequence of the elements, with a few exceptions, correlates to the increasing mass of each atom.
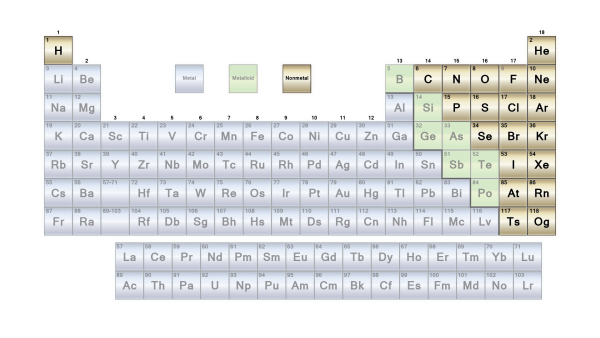
Metals and non-metals are the most common classifications, however the border between the two is blurry. Metals are typically excellent conductors of electricity and heat. The metals are divided into subgroups based on the similarities in their physical and chemical properties. According to the Los Alamos National Laboratory, our periodic chart incorporates generally recognized groups of elements.
Different Types of Elements
The elements are the simplest substances. They can all be classified as either metals or non-metals. Here 7 different types of elements you need to know.
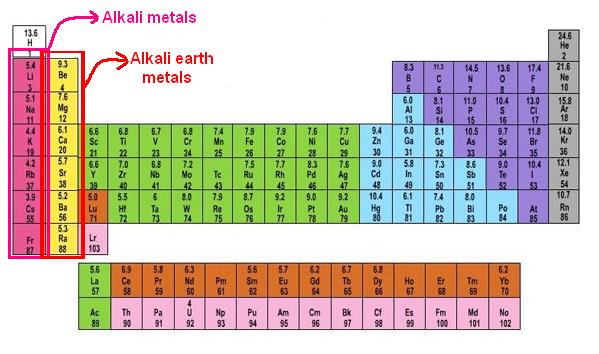
1. Alkaline-Earth Metals
The alkaline-earth metals, which range from beryllium (Be) to radium (Ra), make up Group 2 of the periodic table (Ra). The outermost energy level of each of these elements has two electrons, making the alkaline earths reactive enough that they’re rarely encountered alone in nature. They are, however, less reactive than alkali metals. In comparison to alkali metals, their chemical reactions are generally slower and create less heat.
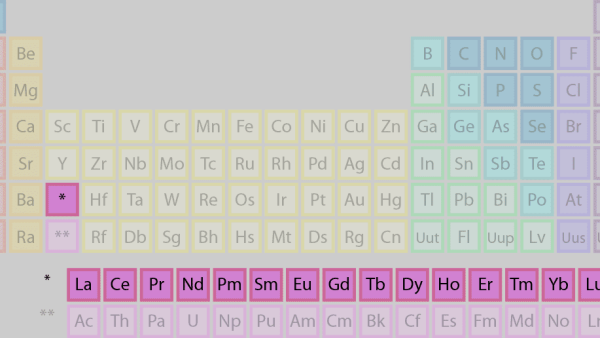
2. Lanthanides
It is separated and flipped sideways to create the top row of the island that floats at the table’s bottom. Lanthanides are elements 57 to 71, ranging from lanthanum (La) to lutetium (Lu) (Lu). This group of elements has a silvery white appearance and tarnishes when exposed to air.
The actinides, which make up the bottom row of the island, are made up of elements 89, actinium (Ac), and 103, lawrencium (Lr). Only thorium (Th) and uranium (U) are found in significant quantities in nature on Earth. They’re all radioactive. The inner transition metals are a category that includes actinides and lanthanides.
3. Transition Metals
Returning to the main body of the chart, the remaining transition metals are represented by Groups 3 through 12. When you hear the word metal, you probably think of elements that are hard yet malleable, bright, and have high conductivity. Many of metal’s biggest hits, including gold, silver, iron, and platinum, may be found here.
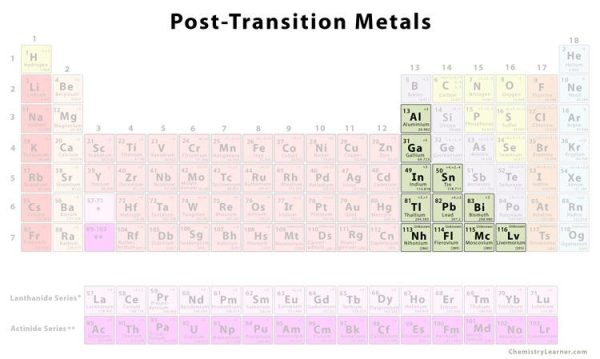
4. Post-Transition Metals
Shared features aren’t neatly separated along vertical group lines prior to the jump into the nonmetal realm. Aluminum (Al), gallium (Ga), indium (In), thallium (Tl), tin (Sn), lead (Pb), and bismuth (Bi) are post-transition metals that range from Group 13 to Group 17. These elements have some of the transition metals’ basic properties, although they are softer and conduct less well than other transition metals. Many periodic tables will have a bolded “staircase” line linking boron and astatine below the diagonal. The post-transition metals are grouped together on the left side of this line.
Elements are the simplest chemical substances in which all of the atoms are identical. We may characterize elements as a group of atoms with the same number of protons in their atomic nuclei. While an element’s atoms all have the same amount of protons, they may have different neutron counts and therefore different masses.
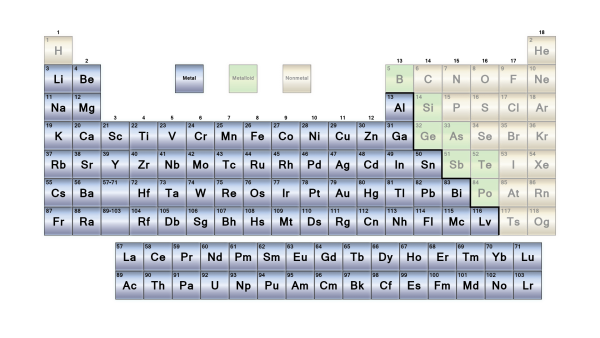
The elements are classified as metallic or non-metallic depending on their groups in the periodic table. Main Group Metals, Transition Metals, and f-block metals are the three types of metallic materials. These are further subdivided based on their characteristics.
5. Metallic Elements
Metals make up the majority of the elements in the periodic table. Alkali metals, transition metals, lanthanides, actinides, and alkaline earth metals are all metals. On a periodic table, metals are separated from nonmetals by a zigzag line that runs from carbon to radon. Phosphorus, selenium, and iodine are the elements that separate the two.
6. Non-Metallic Elements
Non-metals make up a small percentage of the periodic table. These may be found on the periodic table’s right-hand side.
Sulphur, carbon, all halogens, phosphorus, hydrogen, oxygen, selenium, nitrogen, and noble gases are all non-metals.
Non-metals are found to the left of halogens and to the right of metalloids in the periodic table. Noble gases and halogens are commonly referred to as non-metals since they are non-metals as well.
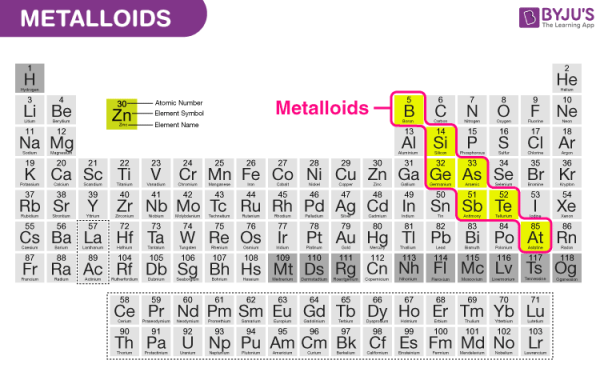
7. Metalloids
Metalloids are a tiny group of elements found in the periodic table of elements between boron and aluminium and the boundary between polonium and astatine, along the zig-zag line that separates metals from nonmetals.
Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te), and Polonium (Po) are metalloids (Po).